Electronic Medical Devices
FDA Approval, Paragon Can Help
We have helped startups to large medical companies develop a wide range of medical devices. When it absolutely has to work, we are the team to get it done.
Getting through FDA requirements requires meticulous planning from day one. It's time-consuming, it's expensive and if you don't start on the right path from the beginning you may find yourself back at the starting line even after you've developed a prototype. You need an expert in your court all the way through this process.
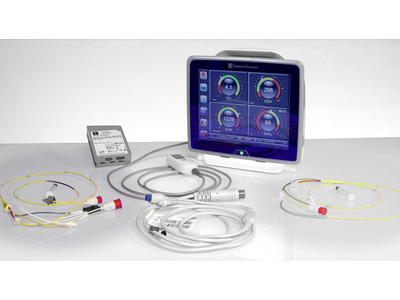
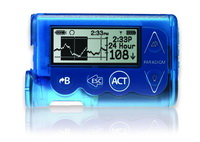
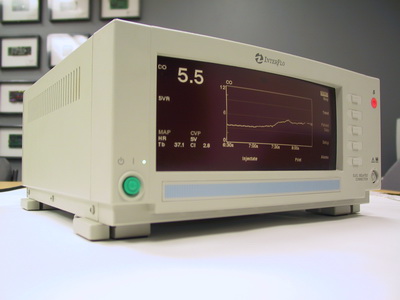
At Paragon Innovations, we have a passion for the design and development of medical electronic device products. Our experience spans years of providing our clients the very best in medical device design and engineering, which has included insulin pumps, cardiac output monitors, pulse oximeters, ultrasonic diagnostics, micro-pulse radar, handheld medical devices, and more. Our goal is to help our clients develop medical device products that are more reliable, higher quality and more cost effective on the most efficient path possible.
Our engineering development team has:
- Extensive background in medical device design
- Passion for customer-focused medical device usability
- Proven experience with verification testing and FDA design documentation requirements
- In the field experience and cross-market knowledge to offer unique design perspective
We are experts in:
- FDA Approval
- ISO 13485 / IEC 60601 / IEC 62304
- Electronic Medical Devices
- Medical Product Development
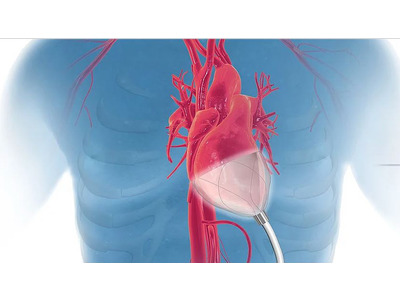
- Cardiac devices
- Diabetes products
- Insulin Pumps
- Infusion Pumps
- Myocardial Protection Devices
- Continuous Cardiac Output Devices
- SvO2
- SpO2
- Electroporation
- Medical Vision Systems
- Invasive Sheath Based Optical Blood Pressure
- Electronic Medical Device
- Medical Product Development
- Electroporation
- High fidelity arterial pressure monitoring
We have been externally audited to ISO 13485 and ISO 9001 compliance, and our medical device design and development experience extends across the entire product development life cycle. We can help you go from product concept all the way to production. Our engineering team can provide a complete turnkey development.
Check our case studies to see some of the medical devices we've developed